Pyrrolizidine alkaloids
es
fr
Pyrrolizidine alkaloids
As one of the leading manufacturers internationally, PhytoLab offers over 1,400 extensively documented herbal reference substances of all classes of natural compounds. Our portfolio currently includes a total of 28 pyrrolizidine alkaloids, 26 pyrrolizidine alkaloid N-oxides and one necine base. Most of our pyrrolizidine alkaloids reference substances are certified as primary reference standards.
Occurrence
Pyrrolizidine alkaloids are common secondary plant metabolites that - until today - have been described in more than 350 plant species. According to chemotaxonomic estimates it is suspected that pyrrolizidine alkaloids occur in more than 6000 plant species from at least twelve higher plant families (Bunchorntavakul C. and Reddy K. R. (2013). Review article: herbal and dietary supplement hepatotoxicity. Alimentary Pharmacology & Therapeutics 37: 3-17; Teuscher E., Melzig M. F., Lindequist U. (2004). Biogene Arzneimittel. Ein Lehrbuch der Pharmazeutischen Biologie. Vol. 6, Wissenschaftliche Verlagsgesellschaft, Stuttgart). It has been estimated that about 3 % of all flowering plants from all continents contain pyrrolizidine alkaloids. More than 660 pyrrolizidine alkaloids and pyrrolizidine alkaloid N-oxides with varying toxic properties have been identified so far. Pyrrolizidine alkaloids are therefore probably the most widespread group of natural substances with toxicological relevance for humans and animals. Plants produce these compounds as a defence against herbivory by insects and mammalians. Due to their widespread occurrence, pyrrolizidine alkaloids are well known contaminants of various foodstuffs such as leaf lettuces, cereals, spices, teas, herbal teas, and honey. Animal feedstuff can be affected as well. The most common source for the pyrrolizidine alkaloids are weeds from the families of the Asteraceae (e.g. genus Senecio, known as groundsel or ragwort), Boraginaceae (most genera) and Fabaceae (e.g. genus Crotalaria, known as rattlepods). About 95% of all pyrrolizidine alkaloids occur in the three aforementioned families as well as in Orchidaceae and Apocynaceae. Honey can be contaminated with pyrrolizidine alkaloids if plants from these and other families are visited by the honey bees.
Chemical structure and classification
From a chemical point of view pyrrolizidine alkaloids are mono- or diesters of 1-hydroxymethyl pyrrolizidine (necine base) and aliphatic mono- or dicarboxylic acids (necic acids). Otonecine-type, platynecine-type, and the C7-diastereomeric retronecine-type and heliotridine-type pyrrolizidine alkaloids are distinguished, their structure depending on the substitution pattern of the necine base.
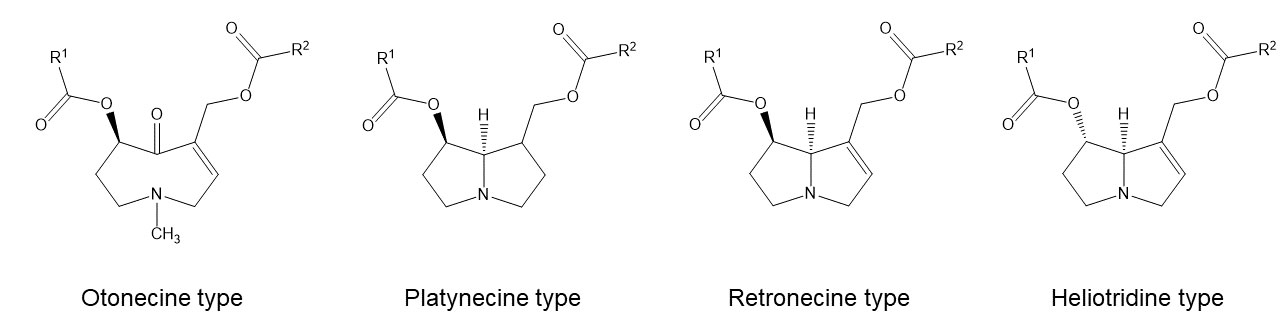
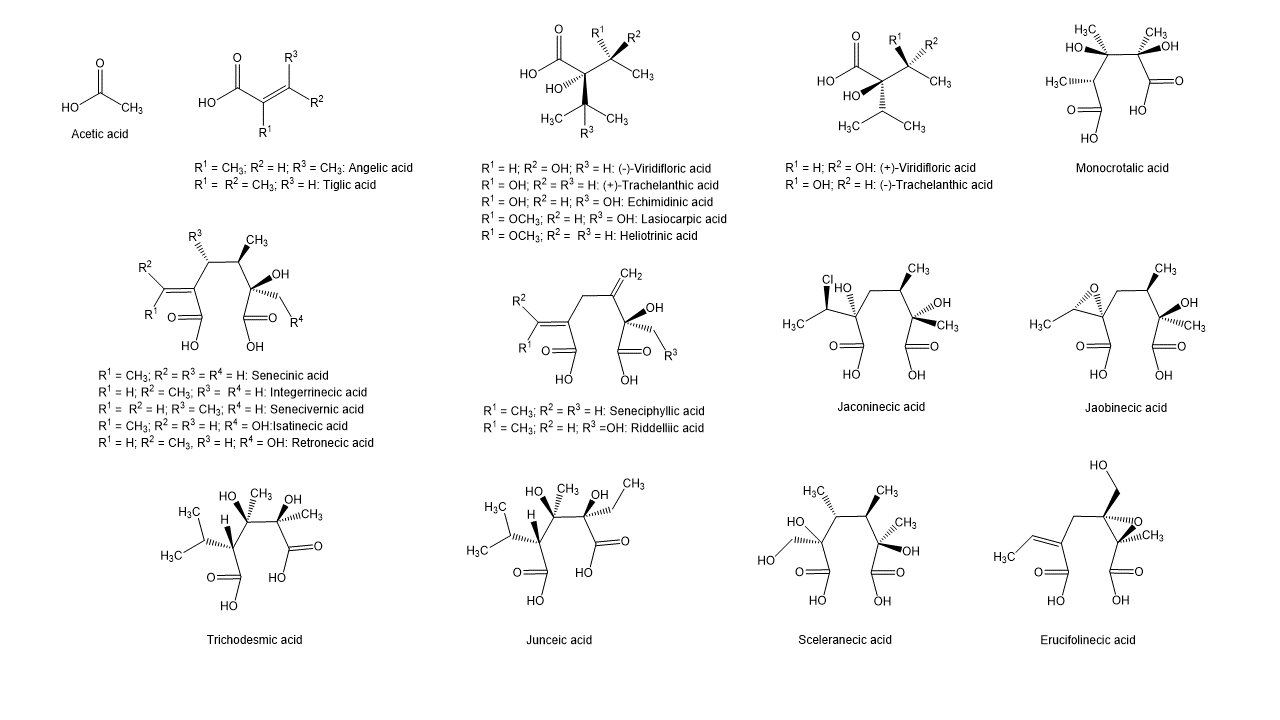
Besides the linear mono- and diesters also cyclic diesters can occur, if esterification occurs with both carboxyl groups of a dicarboxylic acid. Necic acids are typically branched mono- or dicarboxylic acids with a chainlength of 5-10 carbon atoms carrying various substituents. Their names are sometimes related to the corresponding pyrrolizidine alkaloid, such as echimidinic, heliotrinic, integerrinecic, jacobinecic, jaconinecic, junceic, lasiocarpic, monocrotalic, retronecic, riddelliic, sceleranecic, senecinic, seneciphyllinic, senecivernic and trichodesmic acid. Other necic acids are acetic, angelic, isatinecic, trachelanthic and viridifloric acid. Biosynthetically they are derived from amino acids.
Pyrrolizidine alkaloids can also be grouped into subclasses according to their taxonomic occurrence:
- Senecionine-type pyrrolizidine alkaloids are the largest and most diverse class and the group consists of mostly cyclic diesters. Members of this subclass are generic for species of the Asteraceae tribe Senecioneae and are also found in Crotalaria species (Fabaceae)
- Crotalaria spp. also contain monocrotaline-type pyrrolizidine alkaloids that are characterised by eleven-membered ring ring instead of the twelve-membered ring found in cyclic Senecionine-type pyrrolizidine alkaloids
- Lycopsamine-type pyrrolizidine alkaloids are open mono- or diesters. They contain species-specific either retronecine or heliotridine as the necine base. Lycopsamine-type pyrrolizidine alkaloids occur in Asteraceae (tribe Eupatorieae), the Boraginaceae and the Apocynaceae
phyproof® Pyrrolizidine Alkaloid Reference Substances
| Pyrrolizidine alkaloid | Necine base | Necic acid | Overall structure | Subclass |
|---|---|---|---|---|
| 7-Acetylintermedine and N-oxide | Retronecine | (+)-Trachelanthic/Acetic | Open diester | Lycopsamine-type |
| 7-Acetyllycopsamine and N-oxide | Retronecine | (-)-Viridifloric/Acetic | Open diester | Lycopsamine-type |
| Echimidine and N-oxide | Retronecine | Echimidinic/Angelic | Open diester | Lycopsamine-type |
| Echinatine and N-oxide | Heliotridine | (-)-Viridifloric | Monoester | Lycopsamine-type |
| Echiumine and N-oxide | Retronecine | (+)-Trachelanthic/Angelic | Open diester | Lycopsamine-type |
| Erucifoline and N-oxide | Retronecine | Erucifolinecic | Cyclic diester | Senecionine-type |
| Europine and N-oxide | Heliotridine | Lasiocarpic | Monoester | Lycopsamine-type |
| Heliosupine and N-oxide | Heliotridine | Echimidinic/Angelic | Open diester | Lycopsamine-type |
| Heliotrine and N-oxide | Heliotridine | Heliotrinic | Monoester | Lycopsamine-type |
| Indicine and N-oxide | Retronecine | (-)-Trachelanthic | Monoester | Lycopsamine-type |
| Integerrimine and N-oxide | Retronecine | Integerrinecic | Cyclic diester | Senecionine-type |
| Intermedine and N-oxide | Retronecine | (+)-Trachelanthic | Monoester | Lycopsamine-type |
| Jacobine and N-oxide | Retronecine | Jacobinecic | Cyclic diester | Senecionine-type |
| Jaconine | Retronecine | Jaconinecic | Cyclic diester | Senecionine-type |
| Junceine and N-oxide | Retronecine | Junceic | Cyclic diester | Senecionine-type |
| Lasiocarpine and N-oxide | Heliotridine | Lasiocarpic/Angelic | Open diester | Lycopsamine-type |
| Lycopsamine and N-oxide | Retronecine | (-)-Viridifloric | Monoester | Lycopsamine-type |
| Monocrotaline and N-oxide | Retronecine | Monocrotalic | Cyclic diester | Monocrotaline-type |
| Retronecine | Retronecine | - | necine base | - |
| Retrorsine and N-oxide | Retronecine | Isatinecic | Cyclic diester | Senecionine-type |
| Riddelliine and N-oxide | Retronecine | Riddeliic | Cyclic diester | Senecionine-type |
| Rinderine and N-oxide | Heliotridine | (+)-Trachelanthic | Monoester | Lycopsamine-type |
| Sceleratine and N-oxide | Retronecine | Sceleranecic | Cyclic diester | Senecionine-type |
| Senecionine and N-oxide | Retronecine | Senecic | Cyclic diester | Senecionine-type |
| Seneciphylline and N-oxide | Retronecine | Seneciphyllic | Cyclic diester | Senecionine-type |
| Senecivernine and N-oxide | Retronecine | Senecivernic | Cyclic diester | Senecionine-type |
| Senkirkin | Otonecine | Senecic | Cyclic diester | Senecionine-type |
| Trichodesmine and N-oxide | Retronecine | Trichodesmic | Cyclic diester | Monocrotaline-type |
| Usaramine and N-oxide | Retronecine | Retronecic | Cyclic diester | Senecionine-type |
Toxicity
A prerequisite for the toxicity is an 1,2-unsaturated necine core structure that forms an ester with at least one branched C5-carboxylic acid. Pyrrolizidine alkaloids with these structural characteristics have been found to be genotoxic and carcinogenic in animal experiments. The saturated platynecine-type pyrrolizidine alkaloids are therefore the least toxic. In larger quantities pyrrolizidine alkaloids can also cause acute and chronic liver damage. It was found that the toxicity of monoesters of the hydroxymethyl group of the necine base is enhanced if a second hydroxyl group is attached to C-7 of the necine core. The toxicity is further intensified if a diester involving the second hydroxyl group is formed. The most toxic properties are attributed to cyclic diesters.
Except for otonecine-type pyrrolizidine alkaloids plants usually contain a mixture of pyrrolizidine alkaloids and their N-oxides. A comparable toxicity is attributed to both forms upon oral ingestion as the N-oxides are metabolized by reductases to the underlying alkaloid. Further metabolisation of 1,2-unsaturated pyrrolizidine alkaloids by certain liver enzymes (cytochrome P450 monooxygenases) results in highly reactive pyrrole esters that can form DNA and protein adducts, that are ultimately responsible for the toxic activity.
Legal provisions
Pyrrolizidine alkaloid contamination is a relevant issue equally for herbal medicinal products as for food and feed. Since pyrrolizidine alkaloid contamination is difficult to control, limits cannot be derived solely on the basis of toxicological calculations. Strongly triggered by findings of the German Federal Institute for Risk Assessment (BfR) on pyrrolizidine alkaloid occurrence in tea and herbal/fruit infusions in 2013, occurrence data have been collected both by industry associations and official control labs in order to establish a sufficiently realistic and representative picture of overall pyrrolizidine alkaloid exposure through food and herbal medicinal products. During the same time collectors, growers and producers of herbs/herbal products have put huge effort into root cause analysis and measures for improvement. These efforts have recently enabled regulators to establish sufficiently protective however reasonably achievable pyrrolizidine alkaloid limits for herbal medicinal products (Public statement on the use of herbal medicinal products containing toxic, unsaturated pyrrolizidine alkaloids (PAs) including recommendations regarding contamination of herbal medicinal products with PAs. EMA/HMPC/893108/2011 Rev. 1, 07 July 2021) and for important pyrrolizidine alkaloid-susceptible food commodities/categories (COMMISSION REGULATION (EU) 2020/2040 of 11 December 2020 amending Regulation (EC) No 1881/2006 as regards maximum levels of pyrrolizidine alkaloids in certain foodstuffs. Official Journal of the European Union, 14.12.2020) as shown in the table below.
Limits for pyrrolizidine alkaloids in food according to Regulation (EU) 2020/2040
| Foodstuffs | Maximum level (μg/kg) | |
|---|---|---|
| 8.4 | Pyrrolizidine alkaloids | |
| 8.4.1 | Herbal infusions (dried product) with the exception of the herbal infusions referred to in 8.4.2 and 8.4.4 | 200 |
| 8.4.2 | Herbal infusions of rooibos, anise (Pimpinella anisum), lemon balm, chamomile, thyme, peppermint, lemon verbena (dried product) and mixtures exclusively composed of these dried herbs with the exception of the herbal infusions referred to in 8.4.4 | 400 |
| 8.4.3 | Tea (Camellia sinensis) and flavoured tea (Camellia sinensis) (dried product) with the exception of the tea and flavoured tea referred to in 8.4.4 | 150 |
| 8.4.4 | Tea (Camellia sinensis), flavoured tea (Camellia sinensis) and herbal infusions for infants and young children (dried product) | 75 |
| 8.4.5 | Tea (Camellia sinensis), flavoured tea (Camellia sinensis) and herbal infusions for infants and young children (liquid) | 1,0 |
| 8.4.6 | Food supplements containing herbal ingredients including extracts with the exception of the food supplements referred to in 8.4.7 | 400 |
| 8.4.7 | Pollen based food supplements, pollen and pollen products | 500 |
| 8.4.8 | Borage leaves (fresh, frozen) placed on the market for the final consumer | 750 |
| 8.4.9 | Dried herbs with the exception of the dried herbs referred to in 8.4.10 | 400 |
| 8.4.10 | Borage, lovage, marjoram and oregano (dried) and mixtures exclusively composed of these dried herbs | 1000 |
| 8.4.11 | Cumin seeds (seed spice) | 400 |
Recommendation
For a reliable quantitative analysis of pyrrolizidine alkaloids and their N-oxides well characterized reference substances are essential. Because of great variances in response factors (e.g. in LC-MS analysis) as well as in toxicity, the quantitative analysis of individual pyrrolizidine alkaloids is preferred over a non-selective sum method. Currently we offer a total of 55 pyrrolizidine alkaloids, including pyrrolizidine alkaloid N-oxides and necine bases, all of them supplied together with a comprehensive certificate of analysis.
For up-to-date information on prices and specifications please check the product detail pages below!
Reference Substances
- 7-Acetylintermedine
- 7-Acetylintermedine N-oxide
- 7-Acetyllycopsamine hydrochloride
- 7-Acetyllycopsamine N-oxide
- Echimidine perchlorate
- Echimidine N-oxide
- Echinatine sulfate
- Echinatine N-oxide
- Echiumine
- Echiumine N-oxide
- Erucifoline
- Erucifoline N-oxide
- Europine hydrochloride
- Europine N-oxide
- Heliosupine sulfate
- Heliosupine N-oxide
- Heliotrine
- Heliotrine N-oxide
- Indicine hydrochloride
- Indicine N-oxide
- Integerrimine
- Integerrimine N-oxide
- Intermedine
- Intermedine N-oxide
- Jacobine
- Jacobine N-oxide
- Jaconine
- Junceine
- Junceine N-oxide
- Lasiocarpine
- Lasiocarpine N-oxide
- Lycopsamine
- Lycopsamine N-oxide
- Monocrotaline
- Monocrotaline N-oxide
- Retronecine
- Retrorsine
- Retrorsine N-oxide
- Riddelliine
- Riddelliine N-oxide
- Rinderine
- Rinderine N-oxide
- Sceleratine
- Sceleratine N-oxide
- Senecionine
- Senecionine N-oxide
- Seneciphylline
- Seneciphylline N-oxide
- Senecivernine
- Senecivernine N-oxide
- Senkirkin
- Spartioidine
- Spartioidine N-oxide
- Trichodesmine
- Trichodesmine N-oxide
- Usaramine
- Usaramine N-oxide


